Lyophilized Peptide Storage and Handling Basics (Lab Use)
If you’re working with research peptides Canada, storage and handling is where labs avoid waste. This post covers basic, non-protocol handling principles for lyophilized peptides (freeze-dried material) and what typically ruins samples: heat, moisture, light, and repeated temperature swings.
This is general information for laboratory context, not medical advice.
Quick answer
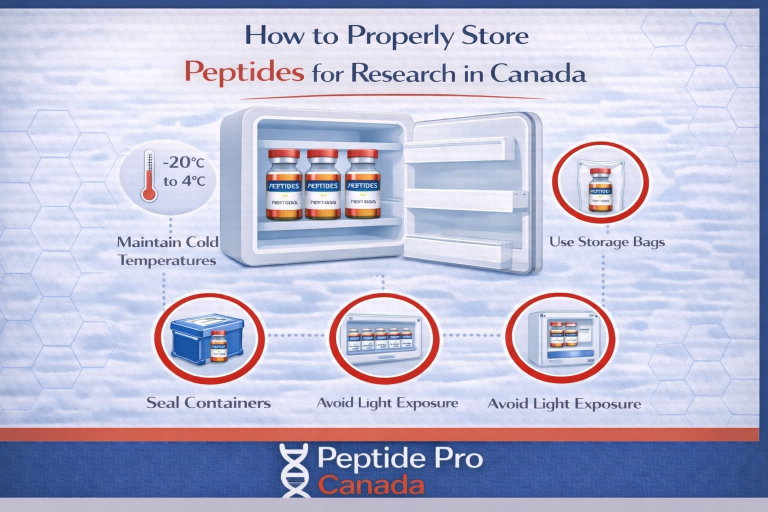
- Keep it dry: moisture is a common cause of degradation in lyophilized material.
- Limit heat exposure: don’t leave vials at room temperature longer than needed.
- Avoid repeat warm/cool cycles: temperature cycling adds stress and can introduce condensation.
- Protect from light: some compounds are light sensitive.
What “lyophilized” means
Lyophilized peptides are freeze-dried. The goal is to reduce water content to improve stability for storage and shipping. Even so, lyophilized material is not indestructible. Heat, humidity, and repeated temperature changes can still impact integrity over time.
The 4 main risks: heat, moisture, light, and temperature cycling
1) Heat exposure
Heat accelerates many degradation pathways. For storage, the basic lab principle is simple: keep samples at appropriate cold storage and minimize time spent warm.
2) Moisture exposure
Moisture is a common problem because it can enter during handling. Condensation can form when cold vials are opened in a warmer, humid environment. A dry workflow is safer than a rushed one.
3) Light exposure
Some compounds can be sensitive to light. If you don’t know the sensitivity profile, a conservative approach is to limit direct light exposure and store in dark conditions when possible.
4) Repeated temperature cycling
Repeatedly warming and cooling material can increase risk of condensation and stress. In labs, a common way to reduce cycling is to plan work so samples spend less time moving between environments.
Handling habits that help (no protocols, just common lab sense)
- Plan your work so the vial is opened for the shortest time practical.
- Keep caps and work surfaces clean and dry.
- Limit unnecessary handling and movement between rooms or storage areas.
- Label and track lot/batch so documentation stays tied to the correct material.
Common mistakes that ruin samples
- Leaving vials in warm areas (sunlight, car, near heat sources).
- Opening cold vials in humid air (condensation risk).
- Repeatedly taking samples in and out of cold storage without a plan.
- Mixing up lot/batch documentation.
Storage notes and expectations (keep it honest)
Different compounds can have different stability profiles. That’s why documentation matters. If you publish COAs, keep them tied to lot/batch and make it easy for researchers to match a COA to the product they received.
FAQ
Do lyophilized peptides “go bad”?
They can degrade over time, especially with heat, moisture exposure, or repeated temperature cycling. Good handling reduces preventable loss.
Is freezing always better?
Cold storage can improve stability for many materials, but stability depends on the compound and how it’s handled. The biggest avoidable issue is often moisture/condensation during handling.
Why does condensation matter?
Moisture can impact stability and can be introduced when cold vials are exposed to warmer, humid environments. A dry workflow helps reduce that risk.
Safe call to action
For documentation, refer to the COA images on each product page (typically the second and third images), and match the COA lot/batch to the product lot/batch when provided.