Research Peptides Glossary: COA, HPLC, LC-MS, Lot, Batch (Plain English)
If you’re buying or selling research peptides Canada, you’ll see the same lab terms everywhere: COA, HPLC, LC-MS, lot, batch, purity, assay. This glossary explains what those words usually mean so you can read product pages and documentation without guessing.
This is general information, not legal advice.
Quick answer
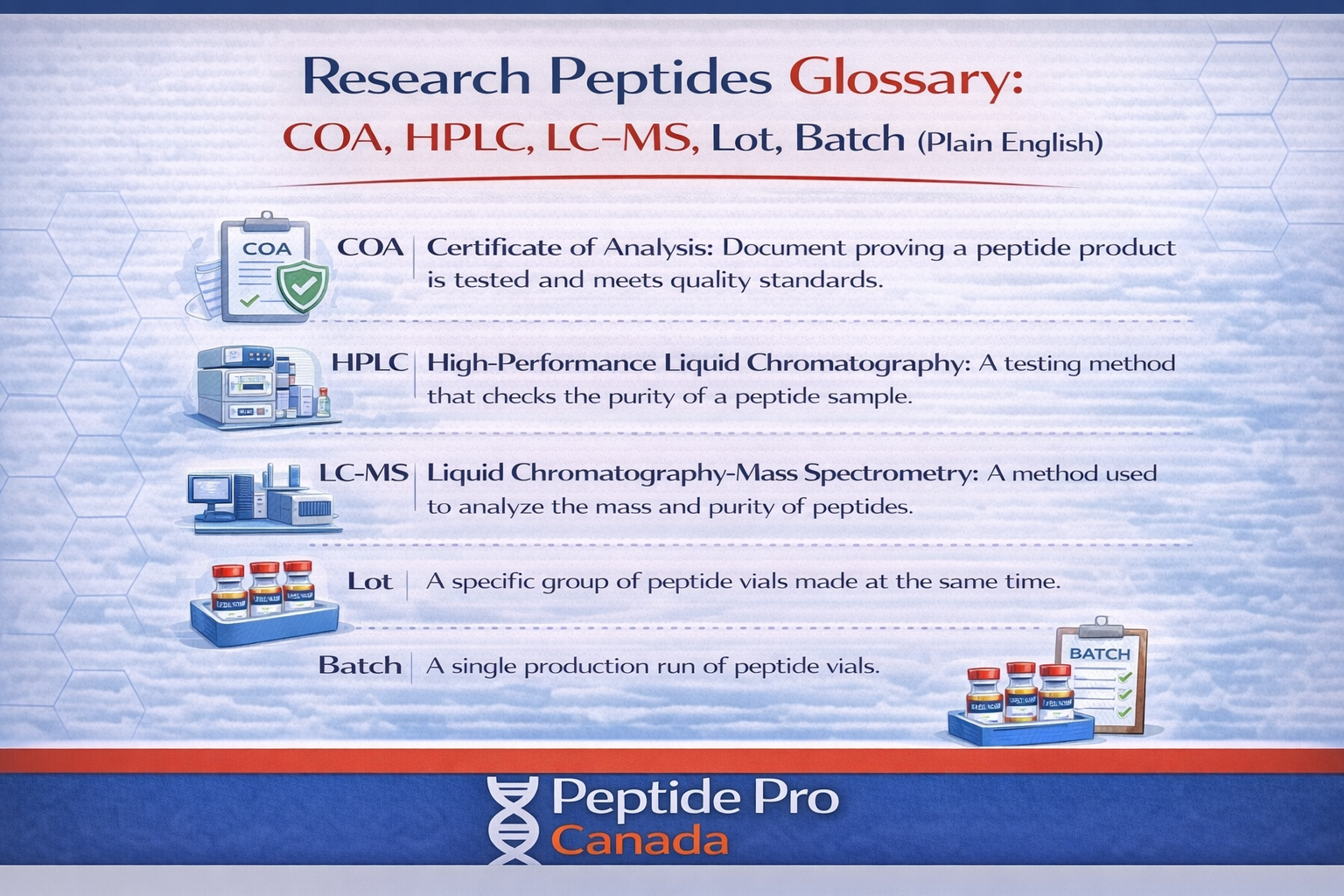
- COA = the batch document that reports test results.
- HPLC = a common method used to report chromatographic purity.
- LC-MS / MS = common methods used to support identity confirmation.
- Lot/Batch = the identifier that ties a COA to a specific production run.
COA (Certificate of Analysis)
A COA is a document tied to a specific lot/batch that lists test results and sometimes specs/acceptance criteria. Strong COAs show:
- product name
- lot/batch number
- test methods used
- results (ideally against a spec)
- lab details and report date
Lot vs batch
These terms are often used interchangeably in online sales. The point is the same: a lot/batch number is the link between the item and the COA. If there’s no lot/batch, the COA has less value.
HPLC
HPLC stands for High-Performance Liquid Chromatography. In peptide COAs, HPLC is commonly used to report a chromatographic purity percentage. It can also be used to profile impurities, depending on the report.
LC-MS / MS
LC-MS stands for Liquid Chromatography–Mass Spectrometry. MS stands for Mass Spectrometry. These methods are commonly used to support identity verification (confirming the material matches the expected molecular profile).
Purity
Purity is often reported as a percentage (frequently from HPLC). People treat this as the only number that matters. It’s useful, but it’s not the whole story.
Assay / content
Assay (sometimes “content”) refers to how much of the target compound is present relative to a standard or spec. It is not the same as purity. Don’t compare an assay number to an HPLC purity number and expect them to match.
Identity
Identity means the testing supports that the material matches what it’s labeled as. COAs may show identity support via MS/LC-MS or similar methods. A COA that doesn’t say how identity was checked is weaker.
Impurities
Impurities are non-target components detected in testing. Some COAs list specific/unspecified/total impurities. Others don’t include impurity detail at all.
Spec / specification
A spec is the acceptance threshold (example: “≥98.0%”). A result matters most when it’s shown against a spec. “Pass” without a spec gives you less to evaluate.
Re-test date vs expiry
Some documentation uses “re-test date” language to indicate when material should be re-evaluated. “Expiry” language varies by vendor and context. The key point for researchers is traceability and clear documentation, not marketing-style dates.
FAQ
What’s the most important part of a COA?
Lot/batch match + method + results against a spec. If any of those are missing, the COA is weaker.
Why do some COAs show only “Pass”?
Some reports are simplified. It’s harder to interpret “Pass” without seeing the spec and the measured result.
Is HPLC purity the same as assay?
No. They can be related, but they are not the same measurement.
Safe call to action
If you want to read your documentation properly, start with our COA guide and check that the COA lot/batch matches the lot/batch for the product where provided.